https://www.flickr.com/photos/sandbaggerone/shares/1CnSoit5E7/
Some custom painted Olivetti typewriters in a Star Wars theme
Via Chad Kohalyk at https://micro.blog/chadkoh/83449602
https://www.flickr.com/photos/sandbaggerone/shares/1CnSoit5E7/
Some custom painted Olivetti typewriters in a Star Wars theme
Via Chad Kohalyk at https://micro.blog/chadkoh/83449602
created: 2026-01-24T11:50:31 (UTC -08:00) tags: [] source: https://www.statista.com/statistics/281488/number-of-deaths-in-the-united-kingdom-uk/ author:
UK annual number of deaths 202<br /> by [[Statista]]<br /> accessed on 2026-01-24T11:50:31
Stop trying to boil the ocean. Focus where impact concentrates.
for - quote - stop trying to boil the ocean - COVID where impacts concentrate
HasUpdates
MSH:Infant,Newborn
IsUpdate
IsUpdate
HasProtocol
LivingReviews:Protocol
IsUpdate
HasUpdate
IsUpdate
Revisar
IsUpdate
Esta no es solo una actualización es una errata, cómo debo tratar esto
LivingReviews:Protocol
https://www.wikidata.org/wiki/Q91945676
LivingReviews:Protocol
Revisar
IsUpdate
LivingReviews:Protocol
LivingReviews:Protocol
IsUpdate 2021
https://hyp.is/0JC6cIdyEfCS6ks5s2WWJQ/pubmed.ncbi.nlm.nih.gov/32511471/
IsUpdate
NoClearUpdate
IsUpdate
IsUpdate
NoClearUpdate
IsUpdate
IsUpdate
IsUpdate
IsUpdate
NoClearUpdate
IsUpdate
HasUpdates
Tiene Updates
ToDo
LivingReviews:Protocol
LivingReviews:Protocol
LivingReviews:Protocol
Tiene updates?
Escrito en italiano
https://www.recentiprogressi.it/archivio/3565/articoli/35458/
LivingReviews/Plataform https://www.deplazio.net/farmacicovid/
Update
NoClearUpdate
Tiene muchas actualizaciones
Update of Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Valk SJ, Piechotta V, Chai KL, Doree C, Monsef I, Wood EM, Lamikanra A, Kimber C, McQuilten Z, So-Osman C, Estcourt LJ, Skoetz N. Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. 189 2 189 0 Similar articles Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Chai KL, Valk SJ, Piechotta V, Kimber C, Monsef I, Doree C, Wood EM, Lamikanra AA, Roberts DJ, McQuilten Z, So-Osman C, Estcourt LJ, Skoetz N. Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. Update in: Cochrane Database Syst Rev. 2021 May 20;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. PMID: 33044747 158 7 56 0 Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, Monsef I, Wood EM, Lamikanra AA, Roberts DJ, McQuilten Z, So-Osman C, Estcourt LJ, Skoetz N. Cochrane Database Syst Rev. 2021 May 20;5(5):CD013600. doi: 10.1002/14651858.CD013600.pub4. Update in: Cochrane Database Syst Rev. 2023 Feb 1;2:CD013600. doi: 10.1002/14651858.CD013600.pub5. PMID: 34013969 Free PMC article. 193 4 81 0 Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Valk SJ, Piechotta V, Chai KL, Doree C, Monsef I, Wood EM, Lamikanra A, Kimber C, McQuilten Z, So-Osman C, Estcourt LJ, Skoetz N. Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. 189 2 189 0 Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Stroehlein JK, Wallqvist J, Iannizzi C, Mikolajewska A, Metzendorf MI, Benstoem C, Meybohm P, Becker M, Skoetz N, Stegemann M, Piechotta V. Cochrane Database Syst Rev. 2021 May 24;5(5):CD015043. doi: 10.1002/14651858.CD015043. PMID: 34029377 Free PMC article. 116 2 88 2 Remdesivir for the treatment of COVID-19. Ansems K, Grundeis F, Dahms K, Mikolajewska A, Thieme V, Piechotta V, Metzendorf MI, Stegemann M, Benstoem C, Fichtner F. Cochrane Database Syst Rev. 2021 Aug 5;8(8):CD014962. doi: 10.1002/14651858.CD014962. Update in: Cochrane Database Syst Rev. 2023 Jan 25;1:CD014962. doi: 10.1002/14651858.CD014962.pub2. PMID: 34350582 Free PMC article. 209 0 97 2
Update
NoClearUpdate
LivingReviews:Protocol
Update. ?
LivingReviews/Plataform www.covid19lnma.com
Update?
LivingReviews:Protocol
Updates
LivingReviews:Protocol
LivingReviews:Protocol
Updates
https://doi.org/10.1136/bmj.o1717 2022
Revisar
LivingReviews:Protocol
LivingReviews:Protocol
Pediatrics
Pediatrics
LivingReviews:Protocol
LivingReviews:Protocol
Pediatrics
Child[MeSH]
Updates https://doi.org/10.1136/bmj.p688 2023
Revisar
he key ingredient that made COVID so unique was a four amino acid sequence inserted into spike protein. So that's 12 nucleotides coding for four amino acids shut down planet earth for a couple of years. That's how powerful this is
for - example - leverage - progress trap - COVID - mix of 4 amino acids inserted into a spike protein
8:55 "did you get vaccinated?" - "yes we did."<br /> wtf? fail.
Absolument. Voici une synthèse détaillée des points clés et des thèmes principaux abordés dans l'extrait d'ARTE Regards, présentée sous forme de briefing :
Briefing : Pédopsychiatrie en Crise Post-Pandémie en Allemagne
Date : 26/10/2023 Sujet : État alarmant de la pédopsychiatrie en Allemagne et conséquences psychologiques de la pandémie sur les enfants et adolescents.
Source : Extraits de "Pédopsychiatrie, le cri d’alarme des médecins | ARTE Regards"
Résumé Exécutif :
Les services sont débordés, les listes d'attente s'allongent dramatiquement et le nombre de jeunes souffrant de troubles psychologiques (dépression, anxiété, troubles alimentaires, phobies sociales, idées suicidaires) a explosé.
Le confinement et les restrictions sanitaires, en privant les jeunes de leurs interactions sociales, de leurs activités et de leurs routines, ont eu un impact dévastateur sur leur développement et leur santé mentale.
Les professionnels de santé tirent la sonnette d'alarme face à une situation jugée inacceptable et redoutent de nouvelles vagues de troubles en cas de futurs confinements.
Thèmes Principaux et Points Clés :
À Hofenbourg, 41 enfants et adolescents étaient en attente au moment du tournage, avec un délai estimé à 6 mois pour une prise en charge.
Les services fonctionnent bien au-delà de leur capacité normale (jusqu'à 110%).
Malgré le besoin urgent, de nombreux patients nécessitant une hospitalisation ne peuvent être admis immédiatement.
Certaines situations extrêmes ont nécessité l'installation de matelas dans les couloirs des urgences pour accueillir de nouveaux patients, y compris ceux en crise suicidaire.
Quote : "l'unité de pédopsychiatrie de la clinique Ander Lindenhur d'Ofenbourg est débordée depuis la crise sanitaire la liste d'attente ne cesse de s'allonger malheureusement ce sont tous des patients qui vont très mal et qui auraient besoin d'être hospitalisés mais on n' pas d'autre choix que de les faire attendre"
Quote : "Depuis le début de l'année nous sommes à 110 % de notre capacité c'est bien au-delà de ce qui est prévu mais nous n'avons pas d'autres choix"
Quote : "il nous est arrivé de recevoir de nouveaux patients alors que nos urgences étaient débordées et il a fallu qu'on installe des matelas dans le couloir"
Augmentation Dramatique des Troubles Psychologiques chez les Jeunes :
On constate une hausse des troubles anxieux et dépressifs, des phobies sociales et des troubles de l'alimentation.
L'augmentation du nombre de patients présentant des symptômes aigus, notamment des idées suicidaires et des tentatives de suicide, est particulièrement inquiétante.
Quote : "partout en Allemagne de plus en plus de jeunes et d'enfants présentent des troubles psychologiques une conséquence directe de la pandémie"
Quote : "résultat une hausse des troubles anxieux et dépressifs mais aussi des phobies sociales et des troubles de l'alimentation"
Quote : "ce qui est d'autant plus inquiétant c'est la hausse du nombre de patients qui en présentent des symptômes aigus c'est-à-dire des personnes suicidaires qui veulent mettre fin à leur jour"
Impact Direct de la Pandémie et des Restrictions Sanitaires :
La fermeture des écoles, collèges, associations et lieux de loisirs a privé les jeunes de leurs interactions sociales essentielles.
L'arrêt des activités (théâtre, scouts, musique, etc.) a enlevé aux jeunes des refuges et des moyens de se ressourcer et de se construire.
Quote : "en raison des restrictions sanitaires les écoles collèges associations périscolaires et autres lieux de loisirs ont fermé leurs portes du jour au lendemain on a dû rester chez nous sans voir nos amis ni nos camarades de classe toutes nos activités se sont arrêtées on nous a arraché à nos vies"
Quote : "la musique c'était mon refuge et même ça on me l'a enlevé certaines personnes ont tout perdu d'un coup leur h et leur loisirs tout ce qui leur permettait de se ressourcer et de reprendre des forces"
Conséquences sur le Développement Social et Émotionnel des Enfants :
Les interactions avec les pairs sont cruciales pour l'acquisition des aptitudes sociales. L'absence prolongée de ces contacts peut avoir des conséquences négatives sur le développement.
Le psychothérapeute Pascal Fischer souligne l'existence de "périodes sensibles" pour l'apprentissage de compétences comme les relations sociales. Manquer ces périodes peut entraîner des déficits durables.
Quote : "il faut éviter de limiter le contact direct entre les enfants pendant trop longtemps ils ont besoin de ces interactions avec des personnes de leur âge parce que c'est comme ça qu'ils acquièrent des aptitudes sociales"
Quote : "Si l'on décide de repousser l'apprentissage d'une de ces compétences il est possible que cette période soit terminée chez l'enfant et qu'il ne puisse tout simplement pas assimiler ses concepts quand un stade a été dépassé on ne peut pas revenir en arrière"
Témoignages de Jeunes et de Leurs Familles :
Amélie (17 ans) : Adolescente active avant la pandémie, elle souffre désormais de dépression et d'idées suicidaires suite au confinement et à la perte de ses activités (scouts, théâtre). Elle attend désespérément une place en hospitalisation.
Sophie (16 ans) : Admise deux fois aux urgences psychiatriques, elle souffre de troubles anxio-dépressifs et d'automutilation suite au harcèlement et à la perte de ses liens sociaux et de son refuge musical pendant le confinement.
Youle (14 ans) : Souffre de dépression et d'automutilation, exacerbated par la pandémie et la perte de ses amis et de sa routine. Elle trouve un soutien dans la thérapie structurée de la clinique.
Ivi (13 ans) : A développé un trouble de l'alimentation (anorexie) pendant la crise sanitaire, liée à l'absence de vie sociale, de routine scolaire et à la perte de repères.
Quote (Amélie) : "un jour ça n'allait plus trop et je ne voulais plus vivre comme ça"
Quote (Sophie) : "pendant le confinement le collège a fermé et tous mes liens sociaux ont disparu... la musique c'était mon refuge et même ça on me l'a enlevé"
Quote (Youle) : "ça a sûrement commencé quand j'ai perdu des amis l'an dernier et ensuite il y a eu la pandémie je me suis sentie seule et paumée"
Quote (Ivi) : "ce qui me manque quand je vais pas en cours c'est de pas pouvoir montrer mes notes... c'est pour cette raison que j'ai commencé à avoir un trouble de l'alimentation"
Méthodes Thérapeutiques Utilisées :
Surveillance constante en soins intensifs pour les patients à risque.
Thérapies individuelles et de groupe.
Exercices de pleine conscience pour la régulation émotionnelle.
Musicothérapie comme moyen de se ressourcer et de combattre les pensées négatives.
Thérapie assistée par l'animal (chiens) pour encourager le mouvement, le plaisir et l'interaction.
Travail sur l'acceptation de soi et du corps (notamment pour les troubles alimentaires).
Ergothérapie (activités créatives comme les mandalas de fleurs) pour travailler ensemble vers un objectif commun.
Mise en place de routines et de structures (horaires de repas, de sommeil, d'activités) pour redonner des repères.
Le Cri d'Alarme des Professionnels de Santé :
Les médecins, comme le Dr. Amélie Fonne Ditourt, ont publié des lettres ouvertes pour alerter le public et les autorités sur la situation intenable.
Ils dénoncent le manque de réaction des différents niveaux de gouvernement.
Ils critiquent le fait que les enfants aient été sacrifiés (en termes de bien-être psychologique) par solidarité envers les plus âgés, sans obtenir de contrepartie pour leurs sacrifices.
Ils redoutent les conséquences de potentiels futurs confinements ou restrictions, notamment la fermeture des écoles, et jugent "scandaleux" que la pression retombe sur les enfants face à l'hésitation des adultes à se faire vacciner.
Quote : "J'ai trouvé que l'impact du confinement en particulier sur les mineurs était trop peu étudié pourtant ils sont en pleine phase de développement et ils traversent bien plus de changements qu'un adulte"
Quote : "La situation est devenue extrêmement difficile on a dû mettre des patients dans le couloir à plusieurs reprises"
Quote : "il y aurait dû y avoir une réaction à tous les niveaux de la municipalité au gouvernement mais ça n'a pas été le cas"
Quote : "pour moi c'est joué avec le feu"
La Solidarité Sacrifiée des Enfants :
Le documentaire souligne que les enfants ont scrupuleusement suivi les consignes, s'isolant et renonçant à leur vie sociale et à leurs activités.
Cette "solidarité" forcée a eu un coût psychologique immense, conduisant à des dépressions sévères et à un sentiment de rejet du monde.
Quote : "c'est par solidarité envers les plus âgés que les enfants ont dû rester chez eux pendant la pandémie au prix d'énormes conséquences psychologiques"
Quote : "J'ai rencontré un grand nombre d'enfants et d'adolescents qui ont suivi les consignes à la lettre ils se sont fait tout petit se sont enfermés dans leur chambre et n'ont plus vu personne ils se sont tellement recroquvillés sur eux-même qu'ils ont atterri ici avec une dépression"
Conclusion :
L'extrait met en lumière une crise majeure de santé mentale chez les jeunes en Allemagne, directement liée aux contraintes de la pandémie.
Le système de pédopsychiatrie est à bout de souffle, incapable de répondre à la demande croissante et urgente.
Les témoignages des jeunes patients et de leurs familles illustrent la souffrance profonde causée par la perte de lien social, d'activités et de routine.
Les professionnels de santé lancent un appel pressant à une prise de conscience et à une action politique pour éviter que cette situation ne dégénère davantage, soulignant les conséquences à long terme pour le développement des jeunes si ces problèmes ne sont pas pris en charge rapidement.
L'attente prolongée pour des soins est particulièrement dangereuse, comme l'illustre le cas d'Amélie dont l'état continue de se dégrader en l'absence d'hospitalisation.
NOTE DE SYNTHÈSE
Sujet : La production sociale des inégalités de santé : Le cas de la vaccination et de la crise COVID-19
Source : Extraits de "La production sociale des inégalités de santé (4) - Nathalie Bajos (2024-2025)" - Intervention de Jérémy Vart
Date : 2024-2025 (selon la source)
Introduction :
S'appuyant sur l'intensification récente des travaux en sciences sociales sur les rapports aux vaccins, l'analyse vise à contextualiser les observations faites pendant l'épidémie de COVID par rapport à d'autres vaccins et aux données antérieures à la crise.
L'exposé s'articule autour de trois paradoxes majeurs qui émergent de l'étude des "rapports ordinaires au vaccin", c'est-à-dire les processus de réflexion et de décision des usagers.
Thèmes Principaux et Idées Clés :
Distinction Attitude/Comportement en matière de vaccination :
Inversement, des personnes favorables à la vaccination peuvent ne pas se faire vacciner en raison d'obstacles (matériels, temporels) ou d'un simple oubli lorsque l'intention n'est pas très forte.
Citation clé : "...de nombreuses nombreuses personnes se font vacciner malgré la persistance de doute fort malgré le fait qu'ils n'ont pas été convaincus de l'utilité de tel ou tel vaccin on l'a notamment vu pendant l'épidémie de COVID-19 après la mise en place du du pass sanitaire..."
Gradient Social des Comportements Vaccinaux (Couverture Vaccinale) : * Le constat d'un gradient social de la vaccination contre le COVID-19 (les personnes en bas de l'échelle sociale étant moins vaccinées) est largement confirmé par de nombreuses études utilisant diverses données (enquêtes par questionnaire, analyses de couverture vaccinale). * Ce gradient n'est pas spécifique au COVID-19 ni à la période récente ; il existe sur d'autres vaccins (comme ROR, grippe) et était déjà observable avant l'épidémie de COVID, bien que les données COVID soient peut-être les meilleures pour étudier ces inégalités. * Ce gradient est également observé dans la plupart des pays qui publient des données sur le sujet, les plus diplômés et les plus aisés étant généralement davantage vaccinés. * Citation clé : "...cette sous-vaccination des publics en bas de l'échelle sociale elle est pas restreinte à la vaccination contre contre le Covid ... On la retrouve sur d'autres vaccins au cours de cette période du Covid..." * Mécanismes Expliquant le Gradient Social des Comportements : * Se faire vacciner nécessite de mobiliser diverses ressources : * Ressources matérielles : coût (même si le vaccin COVID était gratuit, d'autres ne le sont qu'à 70% par la sécurité sociale, laissant la question de l'avance des frais et des complémentaires santé), transport, mais surtout le temps et la disponibilité mentale. Le circuit de vaccination en France, notamment en médecine libérale (prescription, récupération, injection), repose une charge importante sur la personne. * Ressources culturelles : maîtrise des outils d'information, de la langue, connaissance du système de santé. La maîtrise de plateformes en ligne comme Doctolib a favorisé l'accès des plus aisés. * L'offre vaccinale : La manière dont le système de santé prend en compte (ou non) ces différences d'accès est cruciale. Les facteurs favorisant de fortes couvertures vaccinales observés dans d'autres pays incluent : * L'organisation du système de soins autour de centres de santé locaux (guichet unique, association de la vaccination à d'autres prises en charge). * Le développement d'actions ciblées pour les publics éloignés du système de santé (partenariats communautaires, adaptation de la communication, y compris linguistique). * Paradoxe 1 : L'efficacité de la routine institutionnelle (la dissolution de la décision individuelle) : * L'efficacité de la vaccination en centres de santé (comme les PMI en France, fréquentées par des publics plus défavorisés mais dont les enfants sont mieux vaccinés) suggère que la réduction des inégalités passe moins par l'augmentation des ressources des individus pour en faire des "citoyens sanitaires éclairés" que par la simplification et la routinisation de l'acte vaccinal. * Une politique vaccinale est d'autant plus efficace que l'usager a le moins besoin de s'investir. La vaccination par défaut, intégrée à une prise en charge globale et ne nécessitant pas un investissement cognitif important, minimise le rôle de la décision individuelle. * Des études anciennes (comme celles de Marenko et Govedarik sur les PMI de Rennes dans les années 70) ont montré que des publics très défavorisés, peu informés sur les vaccins, avaient leurs enfants très bien vaccinés car l'acte était rendu "évident, voire invisible" au sein d'une prise en charge de confiance et régulière. * Citation clé : "...le succès de la vaccination en centre de santé nous rappelle aussi qu'une politique vaccinale elle est d'autant plus efficace que l'usager a le moins besoin de s'investir dans celle-ci la politique vaccinale elle est jamais aussi efficace que lorsqu'elle est routinisée et que l'usager n'a que peu de décisions à prendre..." * Gradient Social des Attitudes Vaccinales : * Un gradient social des attitudes (défiance, hésitation) à l'égard de la vaccination COVID est également observé dans de nombreuses enquêtes. Ce gradient est fortement lié aux "mauvaises expériences et au manque de confiance à l'égard des acteurs investis dans les politiques vaccinales", comme l'a souligné Nathalie Bajos. * Cependant, ce gradient social des attitudes n'est pas toujours retrouvé sur d'autres vaccins ou dans d'autres pays. Les Baromètres Santé de Santé publique France montrent que les différences d'attitude selon le diplôme et le revenu étaient faibles, voire inexistantes, avant 2009, se renforçant après, et surtout à partir de 2017-2018 et pendant le COVID-19. * Paradoxe 2 : L'acceptation malgré la défiance institutionnelle : * Ce paradoxe réside dans le fait qu'une large part des personnes ne faisant pas confiance aux décideurs politiques et aux autorités de santé "épargnent les vaccins de leur regard critique" ou, du moins, ont une confiance suffisante pour certains vaccins. * Les données d'enquête montrent que même parmi les personnes déclarant ne pas avoir du tout confiance dans les agences de santé, une minorité seulement est défavorable aux vaccins en général, et encore moins à des vaccins spécifiques comme le ROR. * Ce paradoxe renvoie aux analyses de Beck et Giddens sur la confiance dans la modernité : face à la complexité des risques, l'enjeu est de savoir sur quels sujets être vigilant, car il est impossible de se méfier de tout. Les mobilisations, débats publics et l'actualité médiatique jouent un rôle crucial en signalant les sujets "problématiques". * L'émergence de controverses publiques visibles sur les vaccins en France est relativement récente (fin des années 90 pour l'hépatite B, intensification depuis H1N1 en 2009) et semble être un élément essentiel pour que les inégalités sociales se reflètent dans les perceptions vaccinales. * Citation clé : "...une grande partie des personnes qui ne font pas confiance aux décideurs politiques euh et aux autorités de santé épargne les vaccins de leur de leur regardard critique de leurs doutes de leur méfiance..." * La politisation de la vaccination : * Dans les années précédant la crise et pendant celle-ci, les personnalités politiques ont de plus en plus pris part aux débats sur la vaccination, intégrant la question dans la compétition partisane. * Cela se reflète dans les attitudes du public : depuis environ 2018, un gradient politique des rapports aux vaccins est observé. Pendant le COVID-19, les personnes se sentant proches des partis d'extrême gauche ou d'extrême droite, ainsi que celles ne se sentant proches d'aucun parti, ont montré des intentions de vaccination et des taux de vaccination inférieurs à ceux proches du centre (qui montrent une "sur-vaccination"). * Ces différences persistent même en contrôlant d'autres facteurs socio-démographiques (âge, éducation, revenu). La confiance institutionnelle et le degré d'engagement politique sont des facteurs très importants d'hésitation. * Cette politisation redouble les logiques d'inégalité en recoupant les inégalités de santé avec des inégalités politiques. * Le rôle des pratiques informationnelles et le "manque de culture scientifique" : * L'idée courante selon laquelle les doutes s'expliquent par le manque de culture scientifique (un "gradient de compétences culturelles/cognitives") est examinée. * Le diplôme est associé à plus de vaccination et d'attitudes favorables. Des indicateurs de littératie en santé ou scientifique sont également associés à moins d'hésitation vaccinale dans certaines études. * Cependant, ce lien est à tempérer : * Ces variables perdent beaucoup de leur pouvoir explicatif lorsqu'on intègre la confiance dans l'analyse. Les différences d'éducation cachent en partie des différences de niveau d'engagement politique et de confiance institutionnelle. * Le diplôme n'est pas un "bouclier parfait" : l'hésitation vaccinale est répandue, même parmi les plus diplômés (une part significative des Bac+3 et Bac+5 avaient des doutes ou n'avaient pas l'intention de se faire vacciner contre le COVID-19 à certains moments). * Paradoxe 3 : Les ressources culturelles peuvent favoriser la susceptibilité aux discours critiques : * Les femmes ont tendance à être plus hésitantes que les hommes en matière de vaccination. Ceci n'est pas lié à un manque de compétences, mais potentiellement à leur "plus grande propension à s'investir sur les questions de santé", à s'y intéresser, à s'informer auprès de diverses sources. * Ces dispositions, qui devraient en principe favoriser une attitude favorable, peuvent jouer en sens inverse en les rendant plus susceptibles de croiser des discours critiques sur les vaccins et d'y voir un écho (références à des scandales passés, inégalités sociales de santé, etc.). * Il existe des formes "très compétentes" d'hésitation vaccinale. Une logique d'investissement dans la santé ("healthism" ou "santéisme"), combinée à une confiance limitée dans les acteurs de santé publique, peut produire autant de réticence que l'absence d'investissement. * La "désinformation" ne se réduit pas à une distinction claire entre vrai et faux. Les critiques des vaccins imitent souvent les modes de communication scientifique, rendant la frontière floue pour le public. Le choix de croire l'un ou l'autre repose finalement sur la confiance accordée aux institutions et aux types de sources d'information consultées. * Citation clé : "...dans certains cas c'est au contraire le fait même de disposer des ressources euh qui euh favorisent la susceptibilité au discours critique des vaccins..." * Conclusion : Nécessité d'articuler inégalités sociales, pratiques culturelles et transformations médiatiques : * La littérature sur la désinformation s'est trop focalisée sur les connaissances et capacités cognitives, ce qui a conduit à une "dissolution de la thématique des inégalités sociales". * Comprendre la désinformation et le rôle des réseaux sociaux nécessite de replacer ces dimensions dans un cadre d'analyse plus large qui articule les inégalités sociales, les pratiques culturelles (socialement situées, liées aux socialisations de classe, genre, race), et une analyse fine de la transformation des espaces médiatiques (y compris l'émergence de médias mainstream d'extrême droite diffusant de fausses informations). * Ne pas adopter cette perspective risque de retomber sur des "tropes qui assignent le public à l'irrationalité", ce qui renforce le fossé perçu entre décideurs et public et contribue davantage au problème qu'à sa solution.
Points Importants à Retenir :
Implications pour la Prévention et les Politiques Publiques :
Compte Rendu de Séance : La Production Sociale des Inégalités de Santé (3) - Covid-19 et Vaccination
Source: Extraits de "La production sociale des inégalités de santé (3) - Nathalie Bajos (2024-2025)"
Date de la séance: Non précisée (seconde séance après le 3 avril)
Intervenante principale: Nathalie Bajos, sociologue à l'INSERM
Thème central: Les inégalités sociales et les discriminations dans le recours à la vaccination contre le Covid-19.
Points clés abordés:
Introduction et Cadre Problématique:
Enjeux pour la discussion future:
Comment mobiliser les connaissances acquises dans la lutte contre le SIDA pour d'autres épidémies? Comment les politiques de prévention peuvent-elles mieux intégrer la dimension des discriminations et de l'exclusion sociale? Comment construire des discours de santé publique qui résonnent auprès de populations ayant des expériences de défiance vis-à-vis des institutions? L'intervention de Jérémy Vart apportera une perspective plus large sur les questions de vaccination et la spécificité de la vaccination Covid-19.
Note de Synthèse : La Production Sociale des Inégalités de Santé (1) - Nathalie Bajos (2024-2025)
Objet : Synthèse des principaux thèmes et idées clés présentés par Nathalie Bajos lors de la première séance de sa chaire de santé publique sur la production sociale des inégalités de santé, axée sur le cas du COVID-19.
Source : Extraits de la présentation de Nathalie Bajos.
Date de la présentation : 2024-2025 (date précise de la séance non spécifiée, mais fait référence à la leçon inaugurale du 3 avril 2025).
Conférencière : Nathalie Bajos, sociologue et démographe, directrice de recherche à l'INCERM et à l'EHESS. Titulaire de la chaire de santé publique pour l'année 2024-2025.
Résumé :
L'objectif principal est de démontrer comment les inégalités d'exposition au risque de contracter le virus, et par extension de développer des formes graves de la maladie, ne sont pas aléatoires mais sont profondément enracinées dans les structures sociales et économiques.
La présentation met l'accent sur l'importance de l'analyse intersectionnelle pour comprendre comment les rapports de domination de genre, de classe et de race se conjuguent pour créer des vulnérabilités différenciées face à la maladie.
En s'appuyant sur diverses études (quantitatives, qualitatives, analyses d'archives et de recommandations médicales), la conférencière illustre l'ampleur de ces inégalités, notamment selon la profession, la "position raciale" (terme utilisé dans les études citées) et les conditions de vie et de travail.
Thèmes Principaux et Idées Clés :
Une surmortalité pour les catégories sociales les plus défavorisées et les minorités racisées a été confirmée dans tous les pays.
Citations Clés :
Méthodologie et Sources Utilisées :
La présentation s'appuie sur une variété de méthodes et de sources, illustrant la diversité abordée dans la chaire :
Structure des Prochaines Séances :
Les huit séances de la chaire couvriront différentes questions de santé (discriminations, maladies cardio-vasculaires, santé mentale, sexualité, avortement, santé environnementale) en se situant à différentes étapes du processus de production des inégalités, sur différentes populations (population générale, médecins, personnes privées de liberté, zones géographiques spécifiques) et en utilisant différentes méthodes.
La chaire se conclura par un colloque international interdisciplinaire (sociologues, anthropologues, économistes, démographes, épidémiologistes) confrontant modèles théoriques et données empiriques.
Implications pour les Politiques Publiques :
Les travaux présentés visent à "nourrir les politiques publiques la réflexion pour essayer de réduire ses inégalités".
L'analyse des logiques structurelles et l'utilisation d'une perspective intersectionnelle devraient permettre la mise en place de "politiques de prévention beaucoup plus fines et beaucoup plus adaptées" qui ne se limitent pas aux facteurs épidémiologiques mais prennent en compte les déterminants sociaux de l'exposition et de l'accès aux soins. Le manque de données fines est un frein majeur à cette adaptation.
Conclusion :
Cette première séance établit clairement le cadre de la chaire : les inégalités de santé sont un produit social, inextricablement lié aux rapports de domination (genre, classe, race).
L'analyse intersectionnelle est indispensable pour comprendre la complexité de ces inégalités, particulièrement visible dans le cas du COVID-19.
Les conditions de vie et de travail jouent un rôle majeur dans l'exposition au risque, et ces facteurs structurels persistent tout au long du parcours de santé, influençant l'accès aux soins et les issues.
L'approche multidisciplinaire et méthodologiquement diverse de la chaire promet d'éclairer ces mécanismes complexes et de contribuer à une réflexion plus pertinente pour des politiques publiques visant à réduire ces inégalités.
corona war ein intelligenztest. es müssen sowieso 90% sterben global, warum nicht bei den idioten anfangen?
The COVID experience provides a cautionary tale. The unstable economic outlook and higher interest rates meant banks were more cautious about financing some renewable energy projects. And according to the International Energy Agency, small to medium-sized businesses became more reluctant to invest in renewable energy applications such as heat pumps and solar panels.
for - adjacency - economic slowdown - COVID - less investment in renewables
43:50 HIV virus was created in year 1981<br /> 45:18 mysteriously, nature decided to have somebody have sex with a monkey<br /> -> South Park S24E01 The Pandemic Special 7:30 randy fucks a bat in wuhan china
A study published in Nature Climate Change estimated a reduction of 17% in daily emissions in early April 2020 (Figure 1). Greenhouse gas emissions had a reduction of 17 percent from a year earlier on April 7. At the time, China, the United States, India, and other major carbon-emitting countries were all at high levels of quarantine. Overall, daily carbon dioxide emissions decreased by an average of 8.6% between January and April compared to the same period in 2019 (Figure 1).
for - stats - carbon emissions reduction during covid - decarbonization rate - 17% in early April 2020 and 8.6% average between Jan and Apr 2020 compared to same period in 2019
On covid and long term impact on cognition
Vgl [[Wayfinding by Michael Bond]]
"ego investment" ist das problem.<br /> wir mit der impfung seine gesundheit geopfert hat,<br /> der kann nicht zugeben dass die impfung ein fehler war,<br /> der hat falsche hoffnung ("mich trifft es nicht") bis zum bitteren ende.
Charlie Stross provides a personal anecdotal data point to [[Changes in memory and cognition during the SARS-CoV-2 human challenge study]] that revising his writing after covid showed him cognitive issues he didn't realise as he was writing. Comments / responses add to it. Personally I use a type of puzzle and a timer to gauge my concentration and have done for several years. Since my last Covid and in my burnout I now at times don't finish or make mistakes in a puzzle, where I would consistently be in the 5% fastest solvers before.
(Even mild) covid cases are associated with persistent cognitive damage. Empirical data from 2021/2022. Largest diff between measured groups wrt memory and executive functions. No volunteers self-reported cognitive symptoms. Iow covid is associated with cognitive damage, but you won't notice yourself.
Trender et al 2024.
Terapia antiviral para el virus COVID-19: revisión narrativa y análisis bibliométrico
2019 to 2022
3 años
Thus, even where bosses expect attrition, the data shows they get substantially more attrition than they wanted. As a result, 29 percent of these companies are now facing substantial recruitment challenges, as the scale of resignations created serious operational and staffing problems. This misalignment between expectation and reality has left many companies struggling to fill the gaps left by departing employees.
Good. Justice would demand more, but this will probably have to do.
if we get a bird flu mutation causing a human to human viral mutation that, that could cause also a catastrophic outbreak of a pandemic that would exceed, you know, by far what we experienced with COVID 19.
for - bird flu mutation - can exceed impacts of COVID
studies that are coming in right now from the last two years where we were forced to work remotely we see a decrease in Innovation and creative potential in in companies
for - neuroscience research - remote intentional working during Covid - showed decreased productivity and innovation
neuroscience research - remote intentional working during Covid - showed decreased productivity and innovation - Due to only creating intentional work times and eliminating the opportunities for informal meeting - When it is purely intentional work contexts created and no relaxing, informal opportunities to meet, innovation suffers
Excellent performance.
Goosebump-giving
Bitte lasst euch impfen!
But the National Emergency Library (NEL) refutes that.
First time the National Emergency Library is mentioned in the brief.
Drug repurposing for COVID-19 via knowledge graph completion
Reutilización de medicamentos para COVID-19 mediante la finalización del gráfico de conocimiento
Trends in the application of deep learning networks in medical image analysis: Evolution between 2012 and 2020
Tendencias en la aplicación de redes de aprendizaje profundo en el análisis de imágenes médicas: Evolución entre 2012 y 2020
Papeles prometedores de Zingiber officinale roscoe, Curcuma longa L. y Momordica charantia L. como moduladores de la inmunidad contra el COVID-19: un análisis bibliométrico
https://github.com/lmichan/BioDBS
nar:https://academic.oup.com/nar/article/49/D1/D1/6059975
wd:https://www.wikidata.org/wiki/Q107654190
narid:(https://www.oxfordjournals.org/nar/database/summary/2261?action=Search§ion=all&term=litcovid
dc_subject: "COVID-19"
dc_date: 2021
HerramientaPubMed
Résumé de la vidéo [00:00:00][^1^][1] - [00:26:44][^2^][2]:
La vidéo présente une conférence sur l'importance de reconsidérer les rapports entre les sciences naturelles et sociales à la lumière de la pandémie. Elle souligne comment la pandémie a mis en évidence les inégalités sociales et a forcé une réflexion sur notre relation avec le vivant, ainsi que sur l'interdépendance entre les humains et le monde naturel.
Points clés: + [00:00:00][^3^][3] Interdisciplinarité nécessaire * Sciences sociales et naturelles interconnectées + [00:01:14][^4^][4] Impact de la pandémie * Inégalités exacerbées et interaction sociale modifiée + [00:05:34][^5^][5] Conscience écologique et pandémie * Liens entre destruction de la biodiversité et maladies + [00:13:40][^6^][6] Pratiques historiques contre les maladies * Évolution des comportements pour éviter les infections + [00:17:00][^7^][7] Maîtrise des maladies infectieuses * Succès et limites dans le contrôle des microbes + [00:25:43][^8^][8] Vie sociale dans le monde vivant * Sociétés humaines comme extension de la vie sociale animale Résumé de la vidéo [00:26:46][^1^][1] - [00:46:55][^2^][2]: La vidéo aborde les liens entre la biologie et les sciences sociales, en mettant l'accent sur la sociologie des sociétés animales et humaines, l'évolution culturelle et la coévolution gène-culture.
Points clés: + [00:26:46][^3^][3] Connexion biologie-sciences sociales * Sociétés animales comme sociologie ignorée * Importance de l'éthologie et de l'écologie comportementale + [00:35:42][^4^][4] Origines biologiques du culturel * Culture comme solution adaptative * Évolution culturelle parallèle à l'évolution génétique + [00:42:25][^5^][5] Coévolution gène-culture * Impact de la culture sur le biologique * Exemples de coévolution et de construction de niche
Résumé de la vidéo de [00:00:00][^1^][1] à [00:35:00][^2^][2]:
Cette vidéo est un reportage sur le projet Citizen Facts, une initiative de journalisme citoyen qui vise à vérifier les informations diffusées sur le web, notamment sur les sujets liés à la pandémie de Covid-19. Le reportage suit le parcours de plusieurs enquêteurs bénévoles qui s'intéressent aux sources, aux motivations et aux conséquences des fausses informations. Il montre comment ils confrontent leurs recherches aux discours de certains influenceurs controversés, comme Jean-Jacques Crèvecoeur, un youtubeur qui prône la médecine naturelle et qui dénonce le vaccin comme une arme de destruction massive.
Points forts: + [00:00:00][^3^][3] Présentation du projet Citizen Facts * Une plateforme collaborative de vérification de l'information * Plus d'un millier de citoyens européens y participent * Ils enquêtent sur les sujets qui les préoccupent directement + [00:05:06][^4^][4] Enquête sur Jean-Jacques Crèvecoeur * Un youtubeur qui diffuse des informations fausses et dangereuses sur le Covid-19 et le vaccin * Il se présente comme un chercheur de la santé et du bien-être * Il vend des formations de développement personnel inspirées de la méthode Hammer, une pratique médicale contestée + [00:17:17][^5^][5] Rencontre avec Olivia, une admiratrice de Jean-Jacques Crèvecoeur * Une danseuse et comédienne qui partage son discours sur les réseaux sociaux * Elle rejette les résultats de l'enquête qui contredisent son idole * Elle se fie à son intuition et à ses croyances personnelles + [00:23:27][^6^][6] Entretien avec Jean-Jacques Crèvecoeur * Il refuse de reconnaître ses erreurs et ses mensonges * Il accuse les médias mainstream de manipulation et de censure * Il persiste à défendre la méthode Hammer et à dénoncer le vaccin
hah... seit wann lassen sich junkies ihre drogen wegnehmen.<br /> diese leute glauben, sie haben recht und pflicht dass sie sich totspritzen.<br /> weil warum auch nicht, ich seh das als intelligenztest...<br /> vaxx harder!! when it hurts, that means its working!!
we're getting a taste of that in the pandemic yes you adopt a wartime or emergency mindset that helps to liberate a kind of level 00:15:22 of collective purpose it makes new things possible
for: polycrisis wartime mobilization, climate crisis wartime mobilization, 2024 extreme weather - wartime mobilization opportunity
key insight
adjacency statement
The rapid response to the COVID pandemic was a real life case of a wartime scale mobilization in a very short time. This shows that it is possible. We need to see if we can strive for this for climate change. If 2024 becomes the year of extreme weather due to El Nino, then we could use it as an opportunity for a wartime mobilization
one good thing about the COVID pandemic is that it did show that a rapid wartime mobilization is possible, because it did kind of happened during COVID
between middle of March 2020 and the middle of April 2020. 00:44:53 uh four billion people about half the world's population locked down between the middle of March 2020 and middle of April 2020 the concept of social and physical distancing went viral and around the planet and changed 00:45:06 people's behavior all over the planet never has such a large fraction of the human species changed Its Behavior so fast and that was entirely because of the connectivity within the system
for: rapid behaviour change
example - rapid behaviour change: COVID lockdown
question: could we ever imagine the climate crisis or polycrisis having the same impact?
I you know think this is important in the kind of what the left postur is to regime break to system breakdown which 00:35:27 experiencing has to be anti-regime let
for: Lessons from COVID
quote
paraphrase
Thank you. Steve, for raising the alarm on this catastrophe! One minor comment. It should be QC'ed, not QA'ed. Quality control is done first. Quality Assurance (QA) comes after QC. QA is basically checking the calculations and the test results in the batch records. I worked in QC and QA for big pharma for decades. I tried to warn people in early 2021 that there's no way the quality control testing could be done at warp speed. Nobody listened to me despite my decades of experience in big pharma!
"warp speed" sounds fancy, plus "its an emergency, we have no time"...
it really was just an intelligence test, a global-scale exploit of trust in authorities. (and lets be honest, stupid people deserve to die.)
problem is, they (elites, military, industry) seem to go for actual forced vaccinations, which would be an escalation from psychological warfare to actual warfare against the 95% "useless eaters".
personally, i would prefer if they would globally legalize serial murder and assault rifles, then "we the people" would solve the overpopulation. (because: serial murder is the only alternative to mass murder.) but they are scared that we would also kill the wrong people (their servants because they are evil or stupid). (anyone crying about depopulation should suggest better solutions. denying overpopulation is just another failed intelligence test.)
He says that ultimately, about 50% of participants who were screened to be part of the control group couldn’t be included because of continuing symptoms.
Honestly, this should be the headline. A full 50% of people who volunteered to be in the control were actually still suffering symptoms! Half! Of a self-selected group!
food intake and vasoconstriction
Is this why eating is causing instant sleepiness? Non-digestive vessels vasoconstrict and shut off too much cerebral blood flow, then nerves instantly have reduced firing/waste and CO2 build up/diminished mitochondria output/oxidative buildup/ &or then resultant inflammatory triggering cytokine increase?
Vessel endothelial enormous surface area, manipulator of blood flow vasoconstrictor system, and cytokine producer/influencer, and high vulnerability sensitivity to viral infection/corruption...and then it's role or adjacent system and the immediate available Google research on COVID affecting> the vascular elastin system and corrupted elevated production of destructive elastases resulting in reduced vascular compliance then resulting too narrow "pulse pressure" band essentially creating arteriosclerosis.
Also, make sure to be thinking of the entire vascular system not as one system, but subsided by dynamic changing gated sections and inspect signaling creating changing locations and amounts of high/low pressure zones. Also, keep in mind 3 things about BP: 1, when taken with a cuff it is only measuring a reading at the elbow. 2, is a reading from the artery and not giving any direct data from vein part of the system. 3, BP is not the same as blood flow. So I conceive that you could read a good BP, but actual flow could be completely inadequate.
Remember analogy, vascular system is just like car AC system, or any pressurized hydraulic system, or even actually electric circuits. Meaning that there is a high pressure side, the load component(s), and a low pressure side. Also remember veins act as the reservoir tank, and when they constrict it is injecting more blood into the system to, if functioning correctly, allow higher performance and meet increase load demand. It also, therefore has less direct effect on whole system BP vs artery constriction because it's downstream of the load. Arterial constriction conversely has immediate direct effect on systolic BP as it is essentially putting a wall directly downstream of the heart. Therefore, regardless if diastolic pressure is zero or high, when the heart contacts, the pressure shoots straight up.
A working theory component: my pulmonary vein is inappropriately constricting too much. That causes high back up pressure at alveoli. Exercise then induces veinous reservoir injection and increased blood volume into the "working system" further increasing pressure. Possibly arterious had already been fully dilated at rest in order to compensate and then when exercise happens, it can't be dilated further to increase blood flow throughout and BP increases further all behind the pulmonary vein "dam". However it doesn't present as right side heart failure like might initially be guessed (with leg and belly edema) because the right side heart is not failing...yet. So it contains any further backflow and the alveoli are the weakest point and taking the most abuse and pressure is relieved as pulmonary edema. And therefore what may be present is if we look for it, we'll find that actual blood throughput output exiting the heart is too low. And this can exist with a normal ejection fraction because the heart is functioning correctly and pumping the right percentage of what is a low starting volume. And also this can support why right ventricle is showing first signs of enlarging because it's being overpressured and stretching out (enlarging). And this can support why normal BP readings are measured at the arm because it can completely handle the abnormally low blood volume being received in the downstream location it's at. And then therefore this further supports why BP is normal but HR is riding the high limit at rest and then instantly jumps on exertion AND why dizziness happens because the artery system was already maxed out dilation at rest and for any amount of exertion, increasing HR because of the immediate too fast rise in tissue hypoxia due to too low blood supply the brain keeps driving up HR to meet demand. Total result upon exercise: supply continues to more and more not meet demand, HR rises faster and faster to try to inadequately compensate, physically become weaker especially after high output anaerobic every supply deleted in 1-3 minutes and there is no aerobic capacity cavalry with it's O2 rushing in to take over and that's when I fall off the cliff> HR spikes even faster, chest pain immediately jumps, lung edema turns on full tilt as the HR spikes and the resulting pressure is forced to "spray out of the gaskets (alveoli), and brain blood O2 supply immediately becomes super inadequate and the dizziness and need to fall over is the instant result effect. And since dysfuntioning cerebral vasoconstriction is likely the cause or highly involved in migraines, this also supports why the headaches come. ... And perhaps this explaining the rest pain and how it increases with dex and exertion because blood flow o2 becomes inadequate. Then causing lactic acid waste and CO2 buildup... (ie pain). And then it, like all body tissues being deprived necessary blood flow trigger cytokine inflammation response. ... And then, fuck it, maybe this IS chronic fatigue syndrome, and IS long covid explaining PEM, explaining why every symptom imaginable in any combination permutation is being shown, is explaining the observed elevated varing soups of cytokinesis, explaining all variety of tissue damage depending on any person's unique amount of total hit and their particular systems vulnerabilities and ultimately how far down they went on the increasing spiralling cascading systems failure towards total shutdown, and explains why measures at addressing the variety of manifestations are all somewhat helpful, but inadequate and varing efficacy from patient to patient because they are all too downstream of the root cause trunk of the symptom tree where the need to relieve vascular over constriction is the root or next to the root of the symptom tree that is common to all patients. If this were all to be accurate, then the seed would be what caused the break in vasoconstrictor system and repairing/killing it, or perhaps it's a PC bootstrap phenomenon where the simple uncomplex virus was just enough bios code to place innocuous wrenches in any of machines of the systems and then those malfunctioning systems took over control in their new malfunctioning patterns and became the new bosses that are infact the disease, you become your disease, and the initial virus seed has long been killed/departed (they're the ultimate down the road end game that is the totally corrupted bcdhhs that will then exist now as a new monstrous organism slowly lingering and depleting itself and eventually all resources at which point it will have finally killed itself after it destroyed the once thriving self sustaining world it lived in. COVID then is the teenage abusive bf or mean drunk father from their past, that put in motion what would become decades and generations of monsters, years and years after they had been long since gone). And maybe this explains the phasing leaving and returning it symptoms. Because when enough if the symptoms start to be reset/repaired, that starts spiraling the spread of the shutdown of the corruption back to health, but if the spiral up isn't strong enough to overcome the consequential reactive spiral down response, the monster returns and the rebellion is quashed. And so explains why the overall, in every system, stronger less vulnerabilities less armor chinks youth are able to quash with ease the spiral down with their incumbent exceptional spiral up response. .... And aside, this explains why dysautonomia has become a top suspect. And explains why POTS has become almost synonymous with long COVID and CFS.
But since Covid-19, I’ve watched people around me – friends, family and perfect strangers my own age whose stories are told in obituaries – drop dead from this contagion. A sharp sense of existential dread has taken up residence in my psyche. That vague inevitability that I assumed would happen in the distant future smashed me over the head like an anvil in an old cartoon. I could easily die sooner than later. My mortality was, for the first time, in center focus.
D-dimer levels (a fibrin degradation product used to determine the activation of the coagulation cascade), are enhanced in acute COVID-19 cases (Zhou F. et al., 2020; Berger et al., 2020). Different reports have found a correlation between the increase of D-dimer levels and COVID-19 severity with an about 7-fold increase in critically ill patients, associated with an increased mortality risk (Zhou F. et al., 2020; Yao Y. et al., 2020)
Réaliser une étude pluridisciplinaire et longitudinale sur les effets à long terme de la crisesanitaire sur la santé mentale des enfants et des adolescents.
those who got the updated booster had one-tenth the risk of being hospitalized compared with those who are unvaccinated
Basically 65-79 higher seniors are in high risk of being sent to the hospital rather than other people who aren't even vaccines The booster is much more stronger to seniors.
Ga-eun: So many conditions in life are rapidly changing, making even adults feel confused. I hardly dare to im-agine how much fear and anxiety this might cause among children. What about children having to bear all fam-ily problems by themselves? It makes me feel terribly sad. If it was like now, Hana could not even have gone out-side, she would have felt even more lonesome.
Covid quarantines could change how people understand the film due to feelings during lockdowns.
Conservative estimates suggest thatthe failure of timely contact tracing due to the data glitch is associated withmore than 125,000 additional infections and over 1,500 additional COVID-19-related death
La Défenseure des droits recommandeau ministre des Solidarités et de la santéde réaliser une étude pluridisciplinaire etlongitudinale sur les effets à long terme dela crise sanitaire, sur la santé mentale desenfants et des adolescents
Recommandadion 16
Selain itu, isolat virus RaTG13 memiliki nilai kekerabatan 96,1%. Virus ini ditemukan di Yunnan, Cina. Sedangkan isolat virus yang berasal dari tenggiling mempunyai nilai kekerabatan sekitar 91%. Adanya nilai kekerabatan yang tinggi ini dimungkinkan akibat dari evolusi yang telah terjadi dari nenek moyang yang sama.
Untuk diperhatikan, artikel yang dikutip dengan judul "Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak" sudah di erratum 3 tahun yang lalu (https://pubmed.ncbi.nlm.nih.gov/32315626/)
Sehingga masuk dalam jangkauan diskusi PubPeer dengan komentar sebagai berikut:
Readers should become aware of a preprint (DOI: 10.1101/2020.05.07.077016) entitled "The SARS-CoV-2-like virus found in captive pangolins from Guangdong should be better sequenced" that provides critics to the quality of the sequence runs used in Liu et al. 2019 and also in this paper, to infer the genome sequence of Pangolin-CoV.
This preprint states, in particular, that "I found the genome assemblies of GD/P virus of poor quality, having high levels of missing data. Additionally, unexpected reads in the Illumina sequencing data were identified. The GD/P2S dataset contains reads that are identical to SARS-CoV-2, suggesting either the coexistence of two SARS-CoV-2-like viruses in the same pangolin or contamination by the human virus".
Penting bagi penulis untuk menambahkan informasi kebaharuan dan status riset dan temuan terbaru dari artikel yang dikutip. Semoga menjadi koreksi.
Salam.
I asked why she thought that was happening, and she said, “Covid”. The lack of socialization, especially at such a key time in life, had made this incoming class the first one that lacked basic manners and social skills.
Covid has heavily impacted our social/communication skills
forced on the population
at least in the US, no one is being forced to get a vaccine. Not only that, but there's no evidence anyone's even considered it: https://www.snopes.com/fact-check/forced-vaccines-covid-19/
Maybe they're talking about another population?
We analyzed URLs cited in Twitter messages before and after the temporary interruption of the vaccine development on September 9, 2020 to investigate the presence of low credibility and malicious information. We show that the halt of the AstraZeneca clinical trials prompted tweets that cast doubt, fear and vaccine opposition. We discovered a strong presence of URLs from low credibility or malicious websites, as classified by independent fact-checking organizations or identified by web hosting infrastructure features. Moreover, we identified what appears to be coordinated operations to artificially promote some of these URLs hosted on malicious websites.
We found that misinformation-exposure scores are significantly positively related to language toxicity (Fig. 3a; b = 0.129, 95% CI = [0.098, 0.159], SE = 0.015, t (4121) = 8.323, p < 0.001; b = 0.319, 95% CI = [0.274, 0.365], SE = 0.023, t (4106) = 13.747, p < 0.001 when controlling for estimated ideology) and expressions of moral outrage (Fig. 3b; b = 0.107, 95% CI = [0.076, 0.137], SE = 0.015, t (4143) = 14.243, p < 0.001; b = 0.329, 95% CI = [0.283,0.374], SE = 0.023, t (4128) = 14.243, p < 0.001 when controlling for estimated ideology). See Supplementary Tables 1, 2 for full regression tables and Supplementary Tables 3–6 for the robustness of our results.
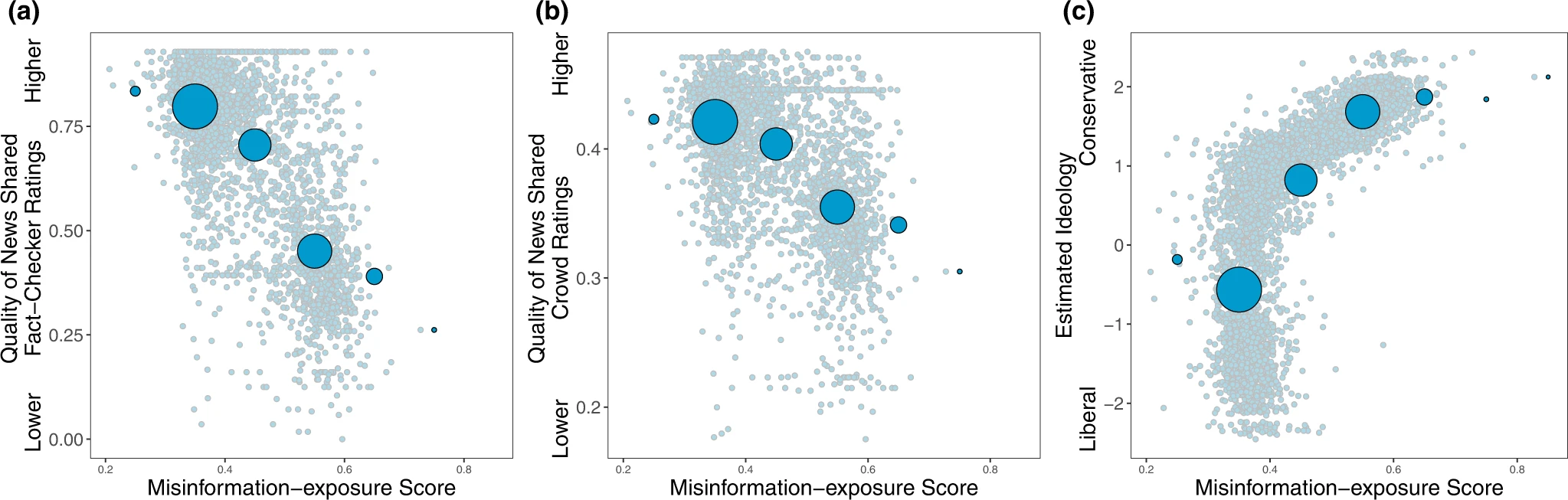
Shown is the relationship between users’ misinformation-exposure scores and (a) the quality of the news outlets they shared content from, as rated by professional fact-checkers21, (b) the quality of the news outlets they shared content from, as rated by layperson crowds21, and (c) estimated political ideology, based on the ideology of the accounts they follow10. Small dots in the background show individual observations; large dots show the average value across bins of size 0.1, with size of dots proportional to the number of observations in each bin.
We find that, during the pandemic, no-vax communities became more central in the country-specificdebates and their cross-border connections strengthened, revealing a global Twitter anti-vaccinationnetwork. U.S. users are central in this network, while Russian users also become net exporters ofmisinformation during vaccination roll-out. Interestingly, we find that Twitter’s content moderationefforts, and in particular the suspension of users following the January 6th U.S. Capitol attack, had aworldwide impact in reducing misinformation spread about vaccines. These findings may help publichealth institutions and social media platforms to mitigate the spread of health-related, low-credibleinformation by revealing vulnerable online communities
we found that social bots played a bridge role in diffusion in the apparent directional topic like “Wuhan Lab”. Previous research also found that social bots play some intermediary roles between elites and everyday users regarding information flow [43]. In addition, verified Twitter accounts continue to be very influential and receive more retweets, whereas social bots retweet more tweets from other users. Studies have found that verified media accounts remain more central to disseminating information during controversial political events [75]. However, occasionally, even the verified accounts—including those of well-known public figures and elected officials—sent misleading tweets. This inspired us to investigate the validity of tweets from verified accounts in subsequent research. It is also essential to rely solely on science and evidence-based conclusions and avoid opinion-based narratives in a time of geopolitical conflict marked by hidden agendas, disinformation, and manipulation [76].
Engagement of religious leaders, for example, has been documented as an important approach to improve vaccine acceptance16,57. Key to the preparation of a COVID-19 vaccine is, therefore, the early and frequent engagement of religious and community-leaders58, and for health authorities to work collaboratively with multiple societal stakeholders to avoid the feeling that they are only acting on behalf of government authorities59.
Similar rates of vaccine hesitance (26% and 25%) and resistance (9% and 6%) were evident in the Irish and UK samples, with only 65% of the Irish population and 69% of the UK population fully willing to accept a COVID-19 vaccine. These findings align with other estimates across seven European nations where 26% of adults indicated hesitance or resistance to a COVID-19 vaccine7 and in the United States where 33% of the population indicated hesitance or resistance34. Rates of resistance to a COVID-19 vaccine also parallel those found for other types of vaccines. For example, in the United States 9% regarded the MMR vaccine as unsafe in a survey of over 1000 adults35, while 7% of respondents across the world said they “strongly disagree” or “somewhat disagree” with the statement ‘Vaccines are safe’36. Thus, upwards of approximately 10% of study populations appear to be opposed to vaccinations in whatever form they take. Importantly, however, the findings from the current study and those from around Europe and the United States may not be consistent with or reflective of vaccine acceptance, hesitancy, or resistance in non-Western countries or regions.
Our kids have lost so much—family members, connections to friends and teachers, emotional well-being, and for many, financial stability at home. And, of course, they’ve lost some of their academic progress. The pressure to measure—and remediate—this “learning loss” is intense; many advocates for educational equity are rightly focused on getting students back on track. But I am concerned about how this growing narrative of loss will affect our students, emotionally and academically. Research shows a direct connection between a student’s mindset and academic success.
“Our kids have lost so much—family members, connections to friends and teachers, emotional well-being, and for many, financial stability at home,” the article begins, sifting through a now-familiar inventory of devastation, before turning to a problem of a different order. “And of course, they’ve lost some of their academic progress.”
These labor actions underscored the frustrations of teachers, who have had to navigate not only the pandemic but also political harangues about their curricula, as well as insufficient pay and other long-standing issues tied to their actual work as educators. Teachers were already leaving the profession, but stress induced by the pandemic accelerated the pace.
https://github.com/lmichan/BioDBS
nar:https://academic.oup.com/nar/article/49/D1/D1/6059975
wd:https://www.wikidata.org/wiki/Q110584966
dc:subject: "COVID-19"
dc:date: 2021
platform to discover potential drugs and targets of COVID-19 at whole transcriptome scale
https://github.com/lmichan/BioDBS
https://github.com/lmichan/BioDBS
wd:https://www.wikidata.org/wiki/Q110577226
narid:https://www.oxfordjournals.org/nar/database/summary/2224
dc_subject:"COVID-19"
dc_date:2021
https://github.com/lmichan/BioDBS
nar:https://academic.oup.com/nar/article/50/D1/D1/6495890
wd:https://www.wikidata.org/wiki/Q110607528
NOTAS:
Falta agregar el artículo de la fuente a wikidata 10.1093/nar/gkab701
https://github.com/lmichan/BioDBS
nar:https://academic.oup.com/nar/article/49/D1/D1/6059975
wd:https://www.wikidata.org/wiki/Q110584892
nar:Aparece en el artículo pero no lo encuentro en la base de datos
dc_subject: "COVID-19"
dc_date: 2021
Many now find Japanese society’s prevalence for mask wearing and the numerous vestiges of preventative measures (social distancing in some restaurants, compulsory temperature checks and hand sanitizer upon entering facilities with cramped quarters, just to name a few) all an overcautious holdover from another time.
lol at westerners thinking wearing masks is overly cautious